They exhibit faster s n 2 reactivity than secondary alkyl halides because the bimolecular transition state is stabilized by hyperconjugation between the orbital of the nucleophile and the conjugated pi bond of the allylic.
Sn2 vinylic halides.
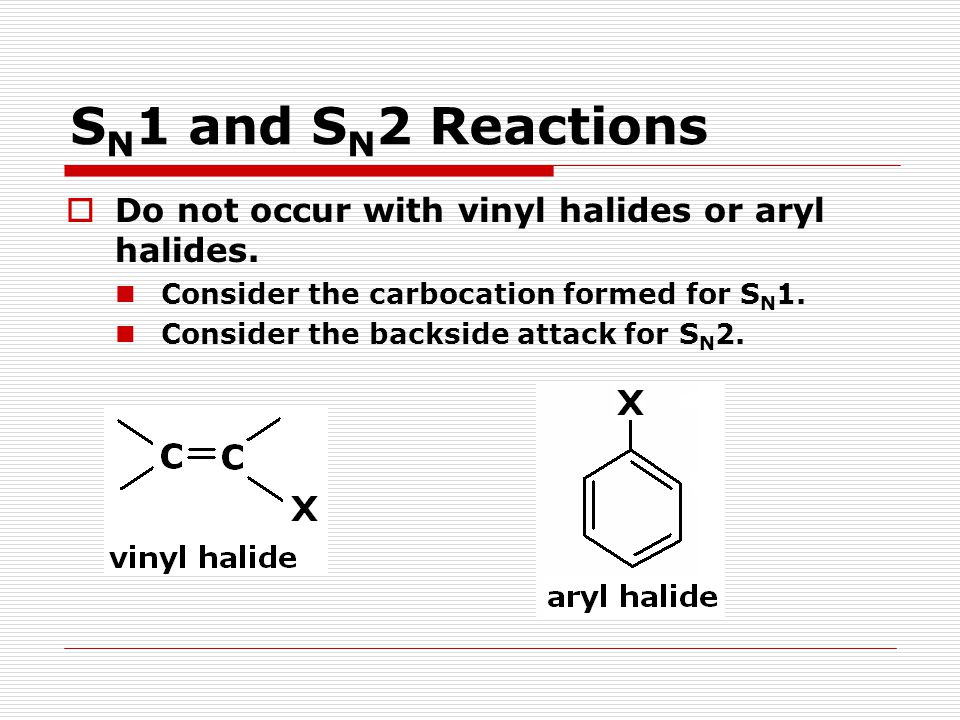
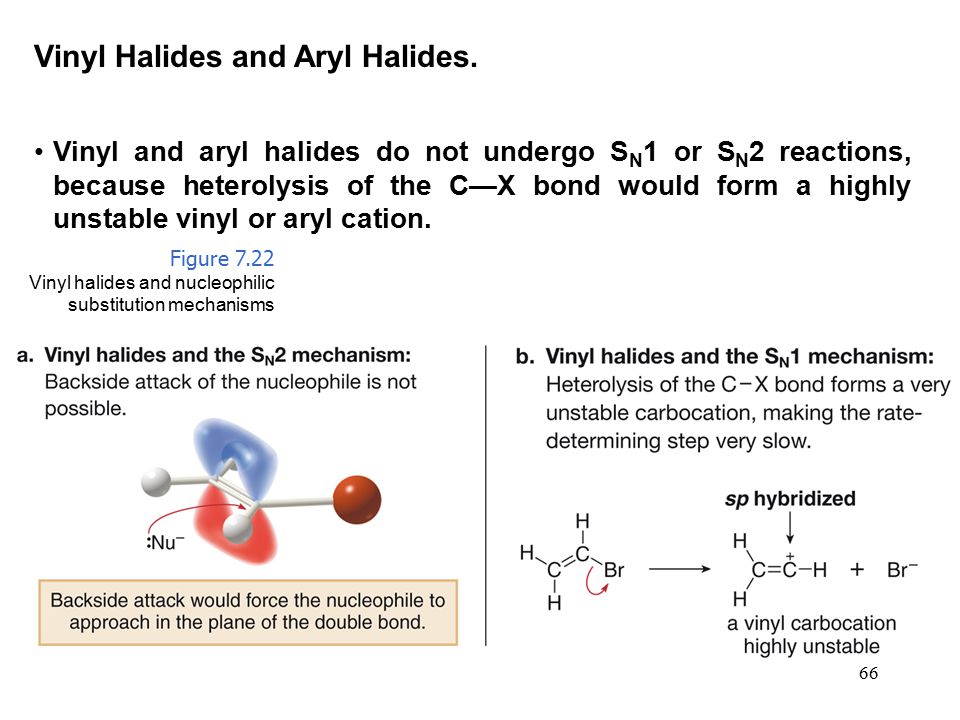
Vinylic and aryl halides however are virtually inert to the conditions that promote s n1 or e1 reactions of alkyl halides.
Haloalkanes haroarenes part 1.
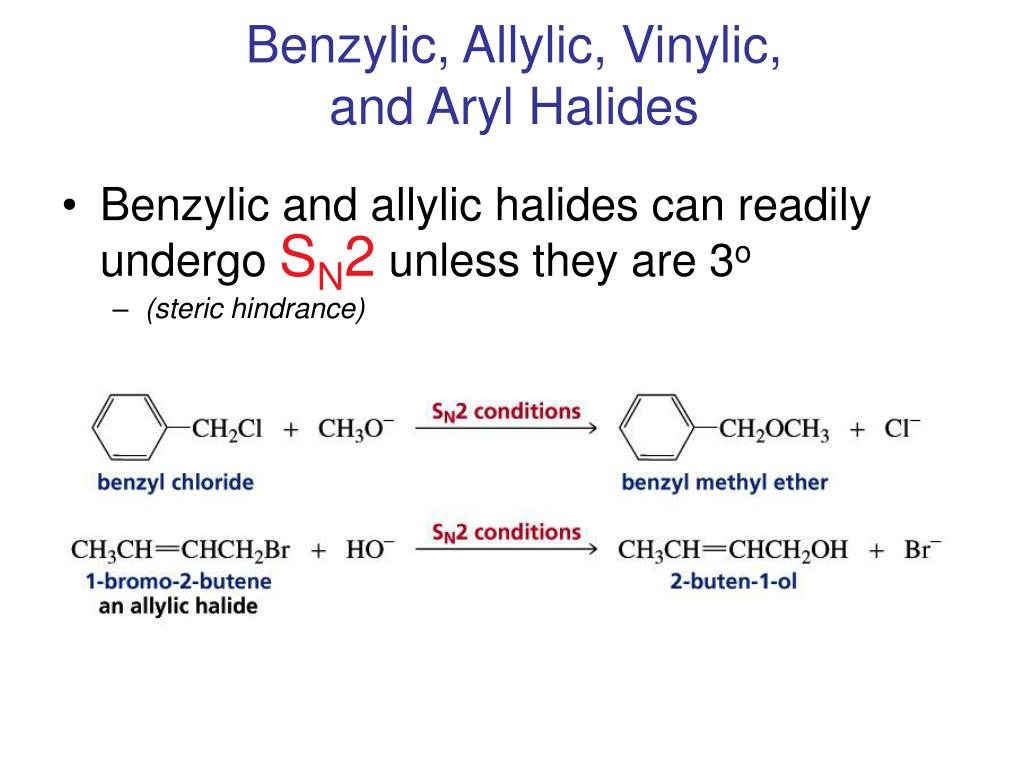
Allylic halides and tosylates are excellent electrophiles for bimolecular nucleophilic substitution reactions s n 2.
There are many cases where allylic halides react preferentially by an mathrm s n 1 process.
The picture below helps explain why this reaction is so much more difficult energetically more costly than the more common solvolysis of an alkyl halide.
We can shift from one mechanism to the.
Chemistry concept 2 058 views.
A sn1 sn2 mechanism on vinyl halide would look like this.
Today i got a good question i want to make a point of posting the best question from the day s teaching and my answer.
Classification allyic vinylic benzylic aryl halides.
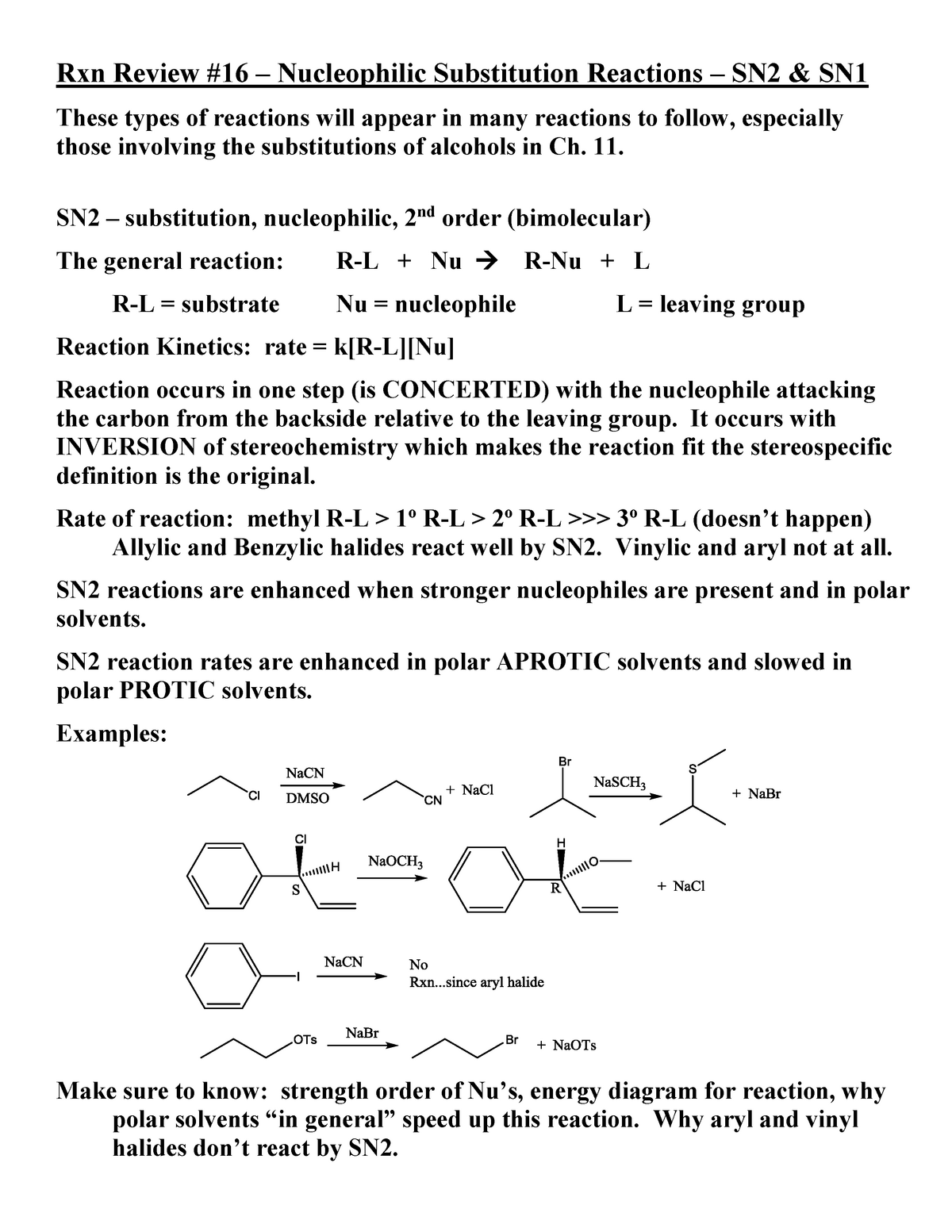
Nucleophilic substitution reactions sn1 and sn2 mechanism.
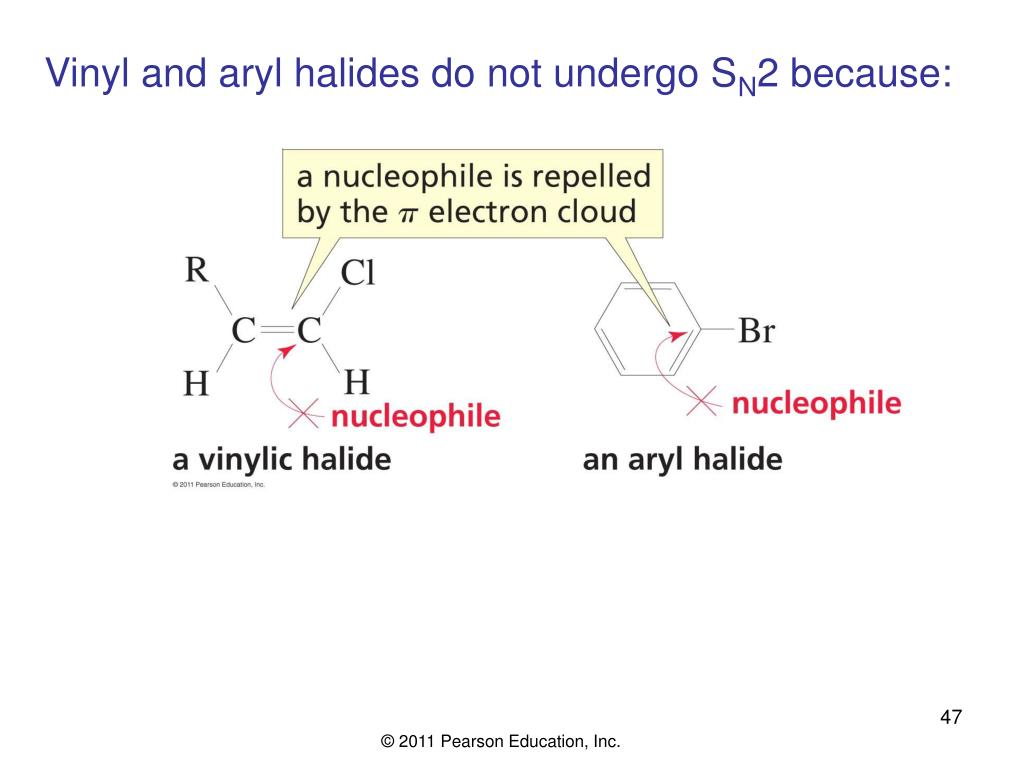
Steric hindrance caused by the benzene ring of the aryl halide prevents s n 2 reactions.
In addition the carbon halogen bond is shorter and therefore stronger in aryl halides than in alkyl halides.
Rapid s n 2 substitution for 1º halides note there are no β.
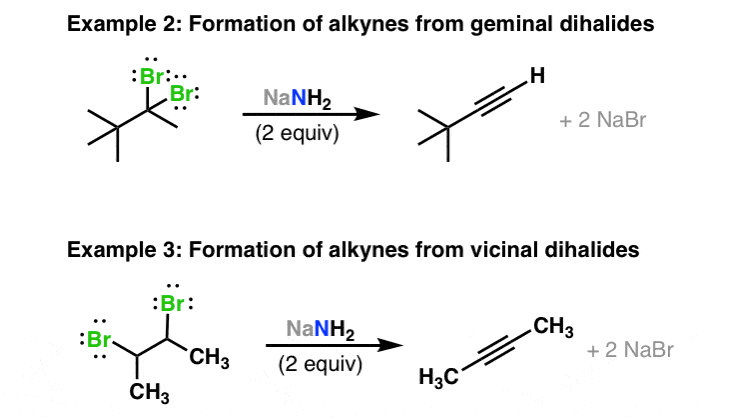
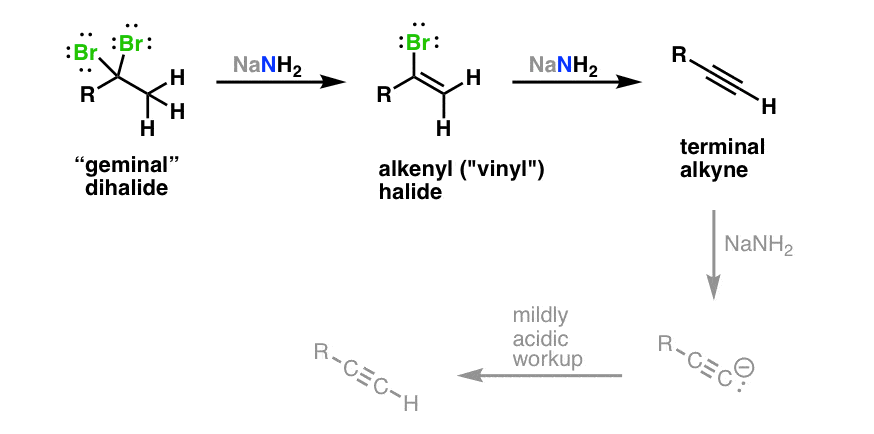
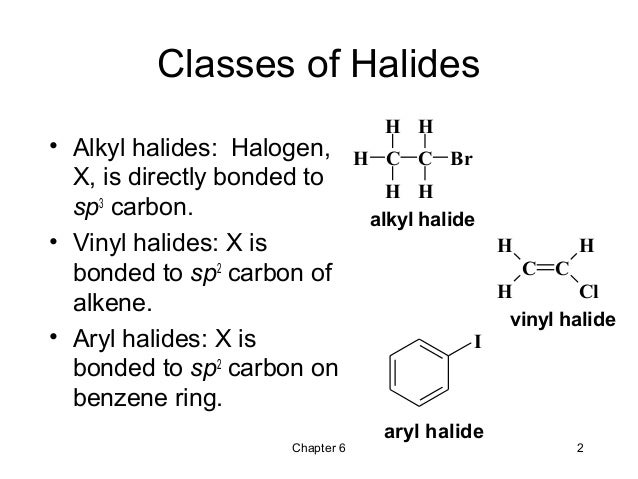
For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides.
In high dielectric ionizing solvents such as water dimethyl sulfoxide acetonitrile s n 1 and e1 products may be observed.
Rapid s n 2 substitution for 1º and 2º halides.
The student asked why do vinyl halides not do the sn2 reaction my answer was that two reasons exist for why the vinyl halide will not react with a nucleophile.
Solvolysis of vinyl halides in very acidic media is an example.
From the perspective of applications the dominant member of this class of compounds is vinyl chloride which is produced on the scale of millions of.
Certain vinylic halides can be forced to react by the s n1 e1 mech anism under extreme conditions but such reactions are relatively uncommon.
The substituents around a double bond are within the same plane therefore an s math n math 2 would give steric hindrance.
A s math n math 2 mechanism is not favoured for 3 reasons.
Since both the allylic mathrm s n 1 and mathrm s n 2 reactions are stabilized there is a delicate balance between the two pathways.
To understand why vinylic and aryl halides are inert under s.
Likewise phenyl cations are unstable thus making s n 1 reactions impossible.
The resultant vinylic carbocations are actually stable enough to be observed using nmr spectroscopy.
S n 2 reactions of allylic halides and tosylates.
In organic chemistry a vinyl halide is a compound with the formula ch 2 chx x halide the term vinyl is often used to describe any alkenyl group.